Clinical Trial Recruitment for T320, a Novel TF-Targeting Anti-Cancer Drug
Release time:
2025-07-09

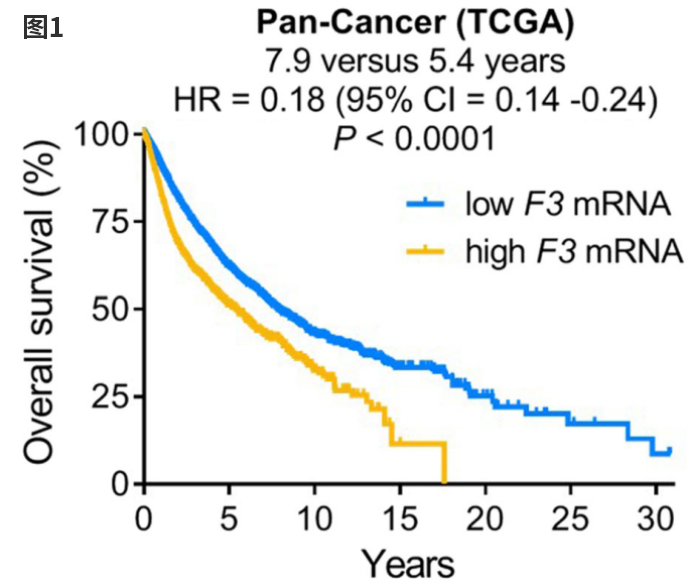
Extensive fundamental research and preclinical studies have confirmed the significant therapeutic value of Tissue Factor (TF) as an emerging target for tumor treatment. TF demonstrates markedly high expression across multiple aggressive solid tumors, including gynecological cancers (cervical, endometrial, and ovarian cancers), urogenital tumors, gastrointestinal cancers (gastric, pancreatic, esophageal, etc.), and lung cancer, among others. In contrast, TF exhibits low or non-exposed membrane protein expression in normal tissues [1]. Studies indicate that TF overexpression in various cancers correlates with poor patient prognosis [2]. Targeting TF can suppress tumor growth, metastasis, and angiogenesis, thereby improving patient survival rates [3]. As shown in Figure 1, the median overall survival in populations with low TF expression is significantly lower than in those with high expression.
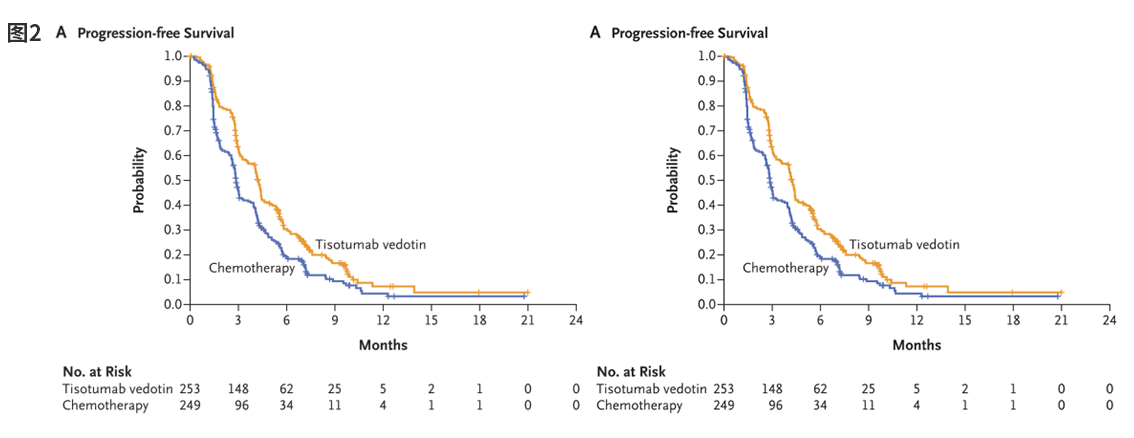
In the field of TF-targeted therapy, breakthrough progress has been achieved with innovative drugs. TIVDAK® (tisotumab vedotin), the world’s first TF-targeting antibody-drug conjugate (ADC), received accelerated approval from the U.S. FDA in September 2021. In April 2024, based on positive Phase III clinical trial results (innovaTV 301), it obtained full approval. As illustrated in Figure 2, study data demonstrate that compared to chemotherapy, TIVDAK significantly prolongs patient survival, reducing the risk of death by 30%, providing a new treatment option for advanced cervical cancer patients [4]. Currently, the drug is undergoing market approval in China and is being investigated in clinical studies for other TF-high-expressing tumors, including ovarian, endometrial, pancreatic, colorectal, and non-small cell lung cancers.
The next-generation TF-targeting drug, T320, also shows promising potential. As a structurally optimized ADC, T320 has demonstrated significant anti-tumor activity in various tumor xenograft models, along with a favorable safety profile. These characteristics position T320 as a highly anticipated new option in TF-targeted therapy, and related clinical trials are currently advancing.
A Phase I clinical trial (Registration No.: CTR20251284) evaluating T320 in advanced solid tumors is now underway at the Chinese Academy of Medical Sciences Cancer Hospital. This study offers eligible patients with recurrent/metastatic pancreatic, cervical, ovarian, endometrial, head and neck, or lung cancers—who have progressed after standard treatments—access to cutting-edge targeted therapy at no cost, under the supervision of a professional medical team.
For participation inquiries or further details, please contact:
Chinese Academy of Medical Sciences Cancer Hospital CRC: Wang Ying |+86 177-3253-9360
Shanxi Provincial Cancer Hospital CRC: Li Na | +86 176-0042-9205
References:
de Bono JS, Harris JR, Burm SM, et al. Systematic study of tissue factor expression in solid tumors. Cancer Reports. 2023;6(2):e1699.
Unruh D, Horbinski C. Beyond thrombosis: the impact of tissue factor signaling in cancer. J Hematol Oncol. 2020;13(1):93.
Hisada Y, Mackman N. Tissue factor and cancer: regulation, tumor growth, and metastasis. Semin Thromb Hemost. 2019;45(4):385-395.
Vergote I, et al. Tisotumab Vedotin as Second- or Third-Line Therapy for Recurrent Cervical Cancer. N Engl J Med. 2024;391(1):[page range].
Tag:
recommend News
Share


